Atlas Antibodies help reveal how redox imbalance drives nerve damage
Cantarero L, Roldán M, Rodríguez-Sanz M, Mathison AJ, Díaz-Osorio Y, Pijuan J, Frías M, Urrutia R, Hoenicka J, Palau F. Abnormal redox balance at membrane contact sites causes axonopathy in GDAP1-related Charcot-Marie-Tooth disease. Res Sq [Preprint]. 2024 Dec 31:rs.3.rs-5682984. doi: 10.21203/rs.3.rs-5682984/v1. PMID: 39801517; PMCID: PMC11722552.
Charcot-Marie-Tooth (CMT) disease, caused by pathogenic variants of GDAP1, results in axonal degeneration, severely affecting motor and sensory nerves. In this study, the researchers focus on the redox imbalance at membrane contact sites, which plays a crucial role in the pathogenesis of GDAP1-related CMT. This imbalance disrupts cellular homeostasis, particularly affecting the mitochondria and endoplasmic reticulum, leading to impaired axonal function and degeneration.
To explore these mechanisms, the study utilized key antibodies from Atlas Antibodies:
The anti-GDAP1 (HPA024334 and HPA014266) Triple A polyclonals were employed in Western blotting (WB), co-immunoprecipitation (CO-IP), and proximity ligation assays (PLA) in both control and GDAP1 knockout mice, helping researchers to identify the role of GDAP1 in regulating mitochondrial and endoplasmic reticulum functions. These assays revealed how the absence of GDAP1 triggers pathological changes at the molecular level.
The anti-S100β (AMAb91038) PrecisA monoclonal was used for multiplex immunohistochemistry and immunofluorescence (IHC-IF) on sciatic nerve sections from both control and GDAP1 knockout mice. This approach provided deeper insight into the axonal changes and the inflammatory response associated with GDAP1 mutations.
Key Findings:
Overall, the research highlights the abnormal redox signaling at membrane contact sites as a critical factor contributing to axonopathy in GDAP1-related CMT, offering potential new therapeutic strategies targeting redox homeostasis to mitigate the disease’s progression.
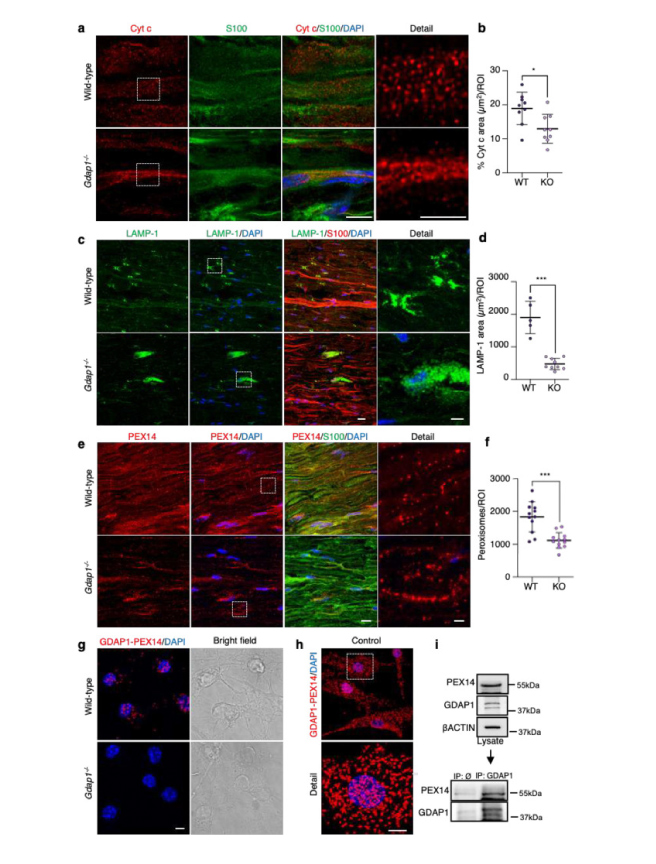
Image from Cantarero L, et al (2024) - Abnormal redox balance at membrane contact sites causes axonopathy in GDAP1-related Charcot-Marie-Tooth disease. a, Representative images of Cytochrome c and S100 staining in sciatic nerve sections from wild-type and Gdap1−/− mice. Magnification details are shown. Scale bar 8 μm, detail 4 μm. b, Quantification of Cytochrome c percentage area per ROI. Mann-Whitney test. (n=9 WT and n=9 KO ROIs, from three animals/genotype). c, Representative images of LAMP-1 and S100 staining in sciatic nerve sections from wild-type and Gdap1−/− mice. Magnification details are shown. Scale bar 15 μm, detail 5 μm. d, Quantification of LAMP-1 total area per ROI. Mann-Whitney test. (n=5 WT and n=9 KO ROIs, from three and five animals/genotype, respectively). e, Representative images of PEX14 and S100 staining in sciatic nerve sections from wild-type and Gdap1−/− mice. Magnification details are shown. Scale bar 10 μm, detail 2 μm. f, Quantification of peroxisome number per ROI. Mann-Whitney test. (n=12 ROI, from three animals/genotype). g, Proximity ligation assay (PLA) between endogenous GDAP1 and PEX14 in control and Gdap1−/− eMNs. Bright field is shown. Scale bar: 10 μm. h, PLA assay between endogenous GDAP1 and PEX14 in control fibroblasts. A detail is shown. Scale bar: 10 μm. i, Co-IP assay of endogenous GDAP1 and PEX14 in control fibroblasts.
All quantitative data are presented as mean ± SD and individual values are displayed as dots. For all comparisons, *p<0.05, **p<0.01, ***p<0.001, ns: not significant. ROI: region of interest.
